FDA Clearance for EyeArt – Eyenuk’s Autonomous AI System for Diabetic Retinopathy Screening
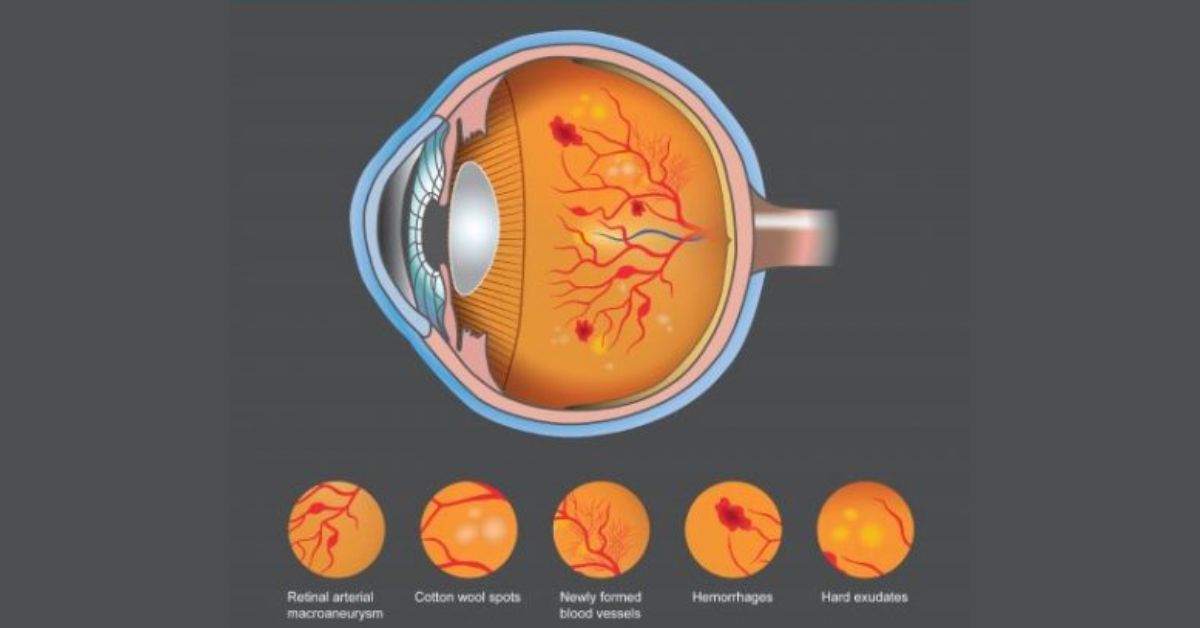
Why Are Diabetic Retinopathy Cases Rising?
Today, diabetes is the leading cause of new cases of adult blindness in the US.
A problem exacerbated by a shortage of eye-care providers which means doctors must focus on treating patients suffering from the disease rather than catching the problem early enough to start effective treatment.
While DR screening is recommended for all diabetic patients, less than half get annually screened due to a lack of eye care specialists to meet the DR screening needs.
Even for those receiving their annual screening, ophthalmology appointment wait times can be weeks or more.
How is AI Being Used for Diabetic Retinopathy Screening?
To address this problem, researchers and vendors have spent years developing AI algorithms that help to accurately detect diabetic retinopathy.
Companies in the US, China, Portugal, and France have created detection algorithms that have produced excellent results in their own clinical trials. However, performance in real-world settings has yet to replicate these positive results.
Researchers at the Veterans Affairs Puget Sound Healthcare System and the Atlanta VA Healthcare System from 2006 to 2018 used the algorithm-based technologies on retinal images from nearly 24,000 veterans.
They found the algorithms’ performance varied when analyzing images from patient populations in Seattle and Atlanta care settings, meaning the algorithms might require additional training on a wider set of images.
Finally on August 05, 2020, an AI-driven solution was approved by th FDA when Eyenuk, Inc. announced that it has received FDA 510(k) clearance to market its EyeArt® autonomous AI System for Diabetic Retinopathy (DR).
The Difference Between AI and Human Detection
Human and AI detection has two key differences:
- Humans have forms of reasoning unavailable to AI
- But AI can detect pixel-level changes in tissue invisible to the human eye
As has been seen in so many studies about AI, the best systems often combine AI-based algorithms with human reasoning. And this is no different for diabetic retinopathy screening. The goal is for AI to augment, not replace, human radiologists to ultimately improve healthcare.
